Medical Device Assembly

Flexible and precision assembly solutions
With innovative technologies and comprehensive knowledge of validation processes, we provide reliable solutions at every stage of product development and production.
Custom Solutions
Our customized solutions can be adapted to individual device characteristics, providing the highest degree of flexibility. With a range of assembly platforms, at our disposal, we optimize the machine design to suit the individual needs of assembly processes, considering production requirements and space constraints in your clean room.

Solutions for Low Output Production (up to 20 PPM)
Compact semi-automatic units designed for functional builds, clinical trials, and early commercial batches. These units offer:
Compact design optimized for lab use
- Full compliance with critical process requirements
- Standard modular bases, with tooling adapted to specific devices
- Variable automation levels depending on component complexity
- Critical processes executed with setups identical to mid-/high output equipment
- CFR Part 11 compliance
Integrated transport systems: free-floating pallet, cam-driven, magnetic.
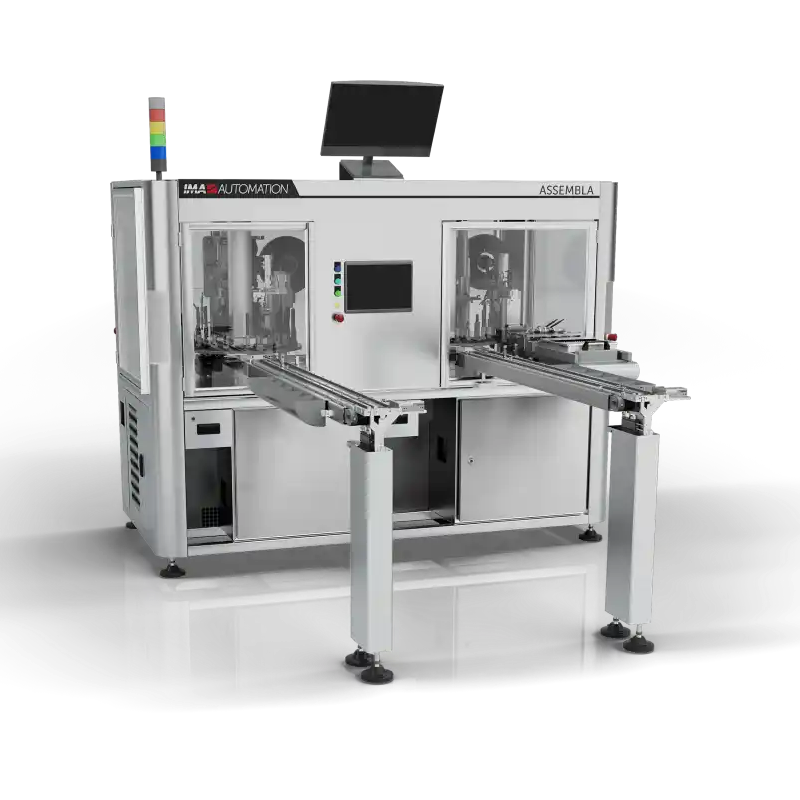
Solutions for Small Batch Production (up to 40 PPM)
Compact cell solutions for small-scale production, adaptable to a wide range of devices with short changeover times. These solutions offer semi-automatic or fully automatic processes similar to those of full-scale production.
- Fully compatible (mechanical, electrical, software interfaces) with high-output platforms
- Excellent accessibility from all sides
- Integration of robots and rotary dials
- Manual, cam-driven, pneumatic, or servo workstations
- Flexible multi-cell lines (master-slave configuration)
- Compatible with Rockwell®, Siemens®, or Beckhoff® control platforms
- CFR Part 11 compliance
Integrated transport systems: free-floating pallet, cam-driven, magnetic.
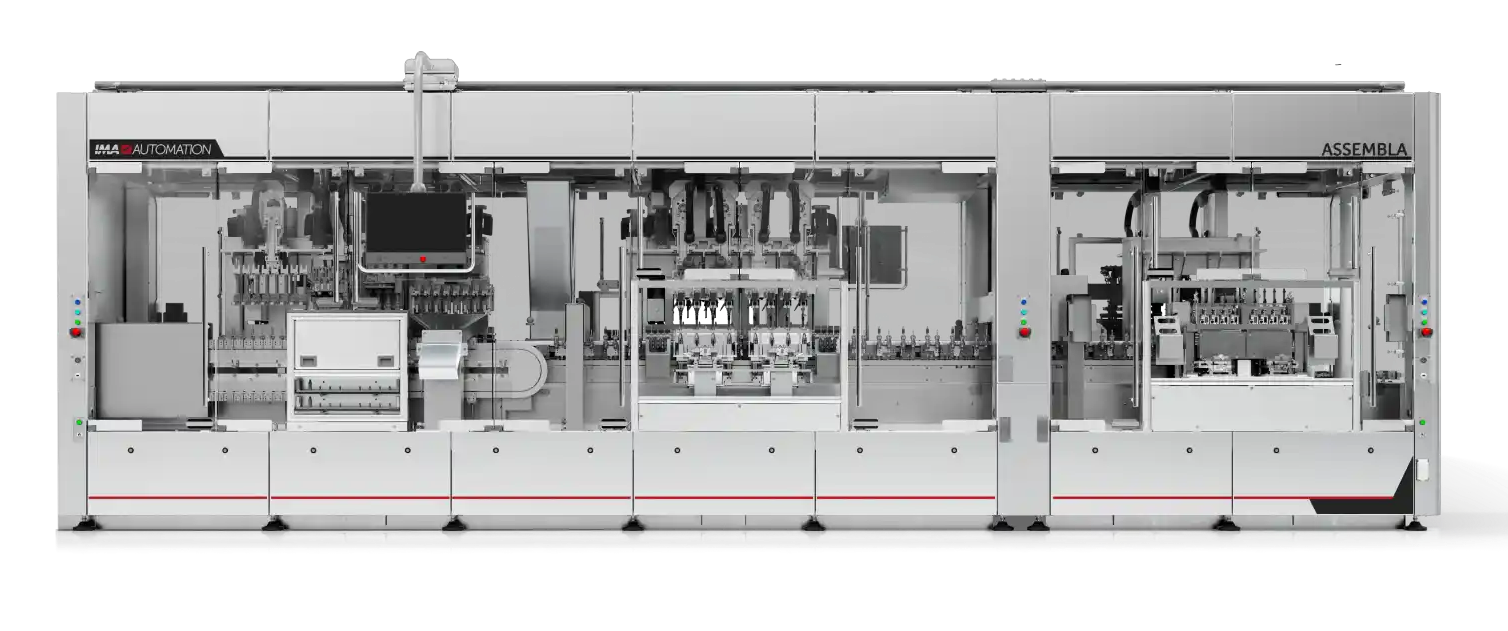
Solutions for High Output Production (up to 200 PPM)
Fully automatic production systems designed to incorporate lessons learned from lower output production. A variety of technologies such as robotics, pneumatics, cam, and servodrives are integrated to maximize performance.
- Fully compatible with high-output platforms
- Single or double-sided platforms
- Robot and rotary dial integration
- Cam-driven, pneumatic, or servo workstations
- Flexible multi-cell lines (master-slave configuration)
- CFR Part 11 compliance
Integrated transport systems: free-floating pallet, cam-driven, magnetic.
Solutions for Super High Output Production (up to 400 PPM)
Designed for large-scale commercial production, these solutions provide high efficiency and seamless process integration, handling outputs up to 400 parts per minute. The streamlined design incorporates automatic material handling to minimize non-value-added tasks, reducing maintenance and energy consumption.
- Linear magnetic transport system for flexibility and speed
- Lean design, incorporating only the required number of tracks for each process
- High efficiency and minimal maintenance
- Single or double-sided platforms
- Low noise levels and no particle generation
- CFR Part 11 compliance
Integrated transport systems: magnetic.

Transport Systems
Transport systems are the heart of robust and functional assembly equipment. Each technology is suited to specific constraints. The wide range of technologies used by IMA meets your challenges.
- FREE FLOATING PALLET
- LINEAR MAGNETIC
- INDEXING/CAM DRIVEN

